Ok, so today I touched up my poster and worked a bit on my reflection paper that is due soon. Not much left at this point. Just have to wait until thursday and then, PRESENTATION TIME!! (is upon us).
I learned a whole lot on this subject, but I still need to become a little more fluently knowledgeable about the classification system, founded by Annie Jump Cannon. It classifies based on star temperature, using the letters OBAFGK and M, O being the hottest, M the coolest.
Followers
Tuesday, May 29, 2012
Monday, May 28, 2012
It's Finished!
Huzzah! My poster is done!! That took a while. I can't believe it's almost time to be done and present. It seems like yesterday I was just starting! Oh, well. And now the year is ending as well and sooo many new projects are popping up. Thank goodness for the three day weekend.
And Happy Memorial Day!!
Also, thanks to the sun for finally coming out and shining on me and my project and stained glass. Also, thanks to all the sites that helped me understand this and the people who helped me a bit. (It's 99% done by me!)
Alas, presentation day is yet to come. Hope I'm prepared!
P.S. Thanks to everyone who actually looked at my blog. It's been fun and I learned a lot!
And Happy Memorial Day!!
Also, thanks to the sun for finally coming out and shining on me and my project and stained glass. Also, thanks to all the sites that helped me understand this and the people who helped me a bit. (It's 99% done by me!)
Alas, presentation day is yet to come. Hope I'm prepared!
P.S. Thanks to everyone who actually looked at my blog. It's been fun and I learned a lot!
Monday, May 21, 2012
Last In-Class Work Day
Though most likely not my last post. Now I'm just working on the layout and construction of my poster board and it's rather astounding how much space there is to fill. I'll manage, though. As soon as I get the chance, I will try to post some picture of my work in progress. It's not very pretty right now, but hopefully that will change. I have a good, clear picture of what I want my poster, at least, to look like now and most of the things I will be putting on it. Since my project works with things in space and non-tangible concepts, it's hard to get a lot of visuals or models, but there are enough things to make a good presentation.
I think there might also be a bit of fun with my nod to the Portal games, since, after all, Aperture Laboratories is funding this research. \( 'O' )/
Aperture Laboratories: A Trusted Friend in Science
I think there might also be a bit of fun with my nod to the Portal games, since, after all, Aperture Laboratories is funding this research. \( 'O' )/
Aperture Laboratories: A Trusted Friend in Science
Monday, May 14, 2012
Finishing Up...
So today I started writing a verbal presentation I can give going over all the research I've done. Hopefully that will keep me from stumbling horribly and looking too nervous (because I assure you, I will).
Unfortunately, it is pretty hard to get my prism experiment to work. My light does not want to make a nice, concentrated beam and my (borrowed) prism is having trouble breaking the light. Hopefully I can get some results, otherwise I'll just talk about how it should work and try to find why mine is not.
(I still have a lot to do on my poster!)
Until next time.
Unfortunately, it is pretty hard to get my prism experiment to work. My light does not want to make a nice, concentrated beam and my (borrowed) prism is having trouble breaking the light. Hopefully I can get some results, otherwise I'll just talk about how it should work and try to find why mine is not.
(I still have a lot to do on my poster!)
Until next time.
Monday, May 7, 2012
More Work!
Today in class we did some more work. I worked on making diagrams of the experiment I did on finding the emission spectra of Hydrogen, Helium, Nitrogen, Neon and Oxygen. Thanks to the chemistry department at my school that was not very hard. The pictures are in another location, but I will hopefully be able to add some soon.
My poster board has yet to be filled, but I'm working on the elements of my poster and a verbal part of my presentation so I can sound (and be) well informed and answer any questions people may have. The presentation setting will be a sort of class symposium or science fair.
My poster board has yet to be filled, but I'm working on the elements of my poster and a verbal part of my presentation so I can sound (and be) well informed and answer any questions people may have. The presentation setting will be a sort of class symposium or science fair.
Monday, April 30, 2012
Experimentation
Today I had some fun searching the Physics classroom for a prism, which I eventually got my hands on. In my previous post, I showed how a simple spectrometer works. For my presentation, I plan on using this recently acquired prism in order to break up white light to show the basic concept. Since I don't have a camera handy right now, here's a picture of another person's prism set up.
 In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result.
In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result.
 In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result.
In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result. Saturday, April 28, 2012
The Spectograph
The tool used by scientists to see and record emission/ absorption spectra is called a Spectrograph or Spectrometer. Modern spectrographs, affixed to telescopes, look something like this:
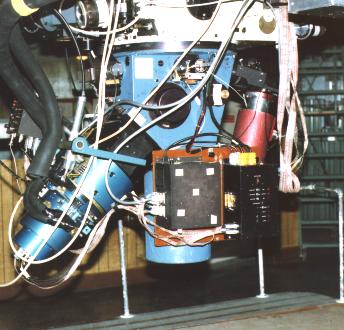





The spectrograph, fitted to the base of the telescope (seen at the top), breaks light into its component colors and records the spectrum. Light from a star goes straight to the collimator at the bottom of the instrument and is reflected back up to a diffraction grating in the middle. It is then reflected down and to the left to a digital detector for storage and display on a video terminal. University of Arizona Steward Observatory spectrograph photo by J. B. Kaler
However, more simple, "primitive" spectrometers involve little more than a prism. Most people know that shining a white light through a prism gives you a little rainbow: (This is because of dispersion).

Similarly, shining condensed light from a burning element, (or bit of the element with an electrical current running through it, as the device in my school's chemistry class does), through a prism will give you the parts of the "rainbow" for that element.
Example:

So, for my project I would like to attempt to make myself a little spectrometer with a prism (and only white light) to display how the technology works. These concepts also have a bit to do with color addition and subtraction, which I have been learning about in physics class recently.
The concept behind emission line and absorption lines goes off of the similar concepts of color addition and subtraction. In color subtraction, certain color wavelengths are absorbed so that others can be reflected. For example, a red shirt absorbs blue and green light and reflects only red. In absorption and emission lines, the electrons absorb certain wavelengths of color and reflect the others.
Color Subtraction:

Color Addition:

The primary light colors are: Red, Blue and Green.
The complementary light colors are: Magenta, Cyan and Yellow.
Emission Spectra
In my final presentation, I plan to explore and display a simple method for classifying elements by their wavelengths. This method is to look at a chemical element's emission spectra. Just like absorption lines, all chemical elements have a particular set of emission lines, which are essentially the opposite of absorption lines. Instead of measuring the energy/ photons absorbed by an electron, scientists measure and mark the energy radiated by electrons when they drop back down to low energies from "kicking" up to high energies. These lines appear as bright lines of color on a dark background instead of black, color-absent lines.
Emission lines are very easy to produce in a laboratory, which is one reason I am presenting them for my project. In the outside world, emissions are produced by street lamps, neon signs and florescent bulbs. In the wonderful world of space, nebulae and some stars can radiate emission lines as well.
Example:

Hydrogen emission lines are radiated by a hot, thin hydrogen gas, and appear at the same wavelengths as the hydrogen absorption lines.
From "Astronomy! A Brief Edition," J. B. Kaler, Addison-Wesley, 1997.
Interesting Find: Whilst looking around the internet for helpful information, I found this great simulation made by (I think) the University of Oregon. It allows you to look at the specific emission and absorption lines for every element on the periodic table and the corresponding wavelengths of each line.
Emission lines are very easy to produce in a laboratory, which is one reason I am presenting them for my project. In the outside world, emissions are produced by street lamps, neon signs and florescent bulbs. In the wonderful world of space, nebulae and some stars can radiate emission lines as well.
Example:

Hydrogen emission lines are radiated by a hot, thin hydrogen gas, and appear at the same wavelengths as the hydrogen absorption lines.
From "Astronomy! A Brief Edition," J. B. Kaler, Addison-Wesley, 1997.
Interesting Find: Whilst looking around the internet for helpful information, I found this great simulation made by (I think) the University of Oregon. It allows you to look at the specific emission and absorption lines for every element on the periodic table and the corresponding wavelengths of each line.
Monday, April 23, 2012
Composition of Stars
As I stated in the earlier post, stars are made up of the same chemical elements as earth, though in different amounts. Most stars are made up of about 90% hydrogen. They also contain helium. The rest of a star's composition is usually oxygen, followed by carbon, neon, and nitrogen. Of the metals stars contain, iron is the dominant element.
But, to put it in proportion, the Sun contains only one atom of oxygen for every 1200 atoms of hydrogen and only one iron atom for every 32 oxygen atoms.
However, there are many stars that do not follow this trend. Depending on their age or position in the Galaxy, it can deviate drastically.
These chemical elements are only discovered from the star's spectra.
But, to put it in proportion, the Sun contains only one atom of oxygen for every 1200 atoms of hydrogen and only one iron atom for every 32 oxygen atoms.
However, there are many stars that do not follow this trend. Depending on their age or position in the Galaxy, it can deviate drastically.
These chemical elements are only discovered from the star's spectra.
Monday, April 16, 2012
Absorption Lines
Now, to get to the roots of my earlier research. After looking at all that information about radiation and temperature, I had to read more about ions, molecules, isotopes and atoms to culminate in Absorption Lines!
In the earlier part of the twentieth century, a discovery in quantum mechanics was made. It was discovered that the electrons that surround an atom have a minimum energy level that they will go to, or a minimum energy level. The electrons naturally seek their lowest energy levels. Since each minimum energy level is different for the electrons surrounding a specific atom or ion, different atoms absorb a specific energy level. However, electrons can be moved from one energy level to another through collisions or the absorption of photons, but an electron in a specific energy level is only capable of absorbing all or none of a photon, it cannot absorb a part of one. As a result, only photons with particular energies, those that correspond to the particular energy levels of the atom, can be absorbed from radiation. Also, since wavelength corresponds to photon energy levels, only specific wavelengths (or colors) can be absorbed.
Thus, since each atom or ion has a different electron structure, only specific wavelengths can be absorbed for that atom or ion.
When one looks at the (visible light/color) spectrum from a hot source (star, celestial body) after it has passed through low-density gas, narrow gaps at particular wavelengths where the light is diminished or even gone altogether are visible. Because of this appearance, these gaps or marks are called "absorption lines." Each atom or ion (of an element) has a unique set of absorption lines.
In stellar spectra, this technique can be used to discover the elements in stars. The deeper parts of stars contain large amounts of very hot, high-density gas and thus tend to radiate all the colors of the spectrum. However, the outer layers of stars are made up of a lower density gas and can act like the low-density gas in the earlier paragraph. Since stars are made up of the same chemical elements on earth (just in larger proportions), they can display an impressive mixture of absorption lines.The radiation from the center of the stars passes through the outer layer of gas allowing for scientists to read the resulting absorption lines. Those lines that are blacked out are the wavelengths that correspond to the elements in radiation.
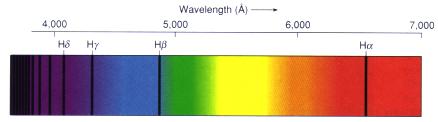
This is an example of the absorption lines of hydrogen against a continuous visual spectrum.
From "Astronomy! A Brief Edition," J. B. Kaler, Addison-Wesley, 1997.
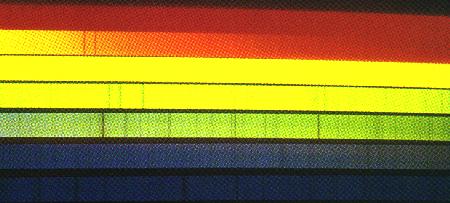
The solar spectrum is filled with absorption lines at particular colors or wavelengths, each dark line associated with a particular atom or ion. The pair in the orange, for example, are made by neutral sodium, the trio in the yellow by magnesium. Kitt Peak National Observatory.
In the earlier part of the twentieth century, a discovery in quantum mechanics was made. It was discovered that the electrons that surround an atom have a minimum energy level that they will go to, or a minimum energy level. The electrons naturally seek their lowest energy levels. Since each minimum energy level is different for the electrons surrounding a specific atom or ion, different atoms absorb a specific energy level. However, electrons can be moved from one energy level to another through collisions or the absorption of photons, but an electron in a specific energy level is only capable of absorbing all or none of a photon, it cannot absorb a part of one. As a result, only photons with particular energies, those that correspond to the particular energy levels of the atom, can be absorbed from radiation. Also, since wavelength corresponds to photon energy levels, only specific wavelengths (or colors) can be absorbed.
Thus, since each atom or ion has a different electron structure, only specific wavelengths can be absorbed for that atom or ion.
When one looks at the (visible light/color) spectrum from a hot source (star, celestial body) after it has passed through low-density gas, narrow gaps at particular wavelengths where the light is diminished or even gone altogether are visible. Because of this appearance, these gaps or marks are called "absorption lines." Each atom or ion (of an element) has a unique set of absorption lines.
In stellar spectra, this technique can be used to discover the elements in stars. The deeper parts of stars contain large amounts of very hot, high-density gas and thus tend to radiate all the colors of the spectrum. However, the outer layers of stars are made up of a lower density gas and can act like the low-density gas in the earlier paragraph. Since stars are made up of the same chemical elements on earth (just in larger proportions), they can display an impressive mixture of absorption lines.The radiation from the center of the stars passes through the outer layer of gas allowing for scientists to read the resulting absorption lines. Those lines that are blacked out are the wavelengths that correspond to the elements in radiation.

This is an example of the absorption lines of hydrogen against a continuous visual spectrum.
From "Astronomy! A Brief Edition," J. B. Kaler, Addison-Wesley, 1997.

The solar spectrum is filled with absorption lines at particular colors or wavelengths, each dark line associated with a particular atom or ion. The pair in the orange, for example, are made by neutral sodium, the trio in the yellow by magnesium. Kitt Peak National Observatory.
Friday, March 30, 2012
A Bit of "Basic" Background
So, a lot of my physics project actually has to do with chemistry, so here's some of the background information from my great information source that I use to help me through:
Source: http://stars.astro.illinois.edu/sow/spectra.html
Photons and Energy:
Though light and its partners can act like waves, at the same time they can act like a stream of particles. In a crude sense, these particles, called "photons," carry the waves. The smaller the wavelength of the photon the more energy it carries, that is, the greater the ability of the photon to act on some physical substance. For example, the energy of infrared is registered as heat, ultraviolet light (the sun) can burn and an X-ray can also cause damage with too much exposure.
Electromagnetic Waves:
Except for the energy they carry, all portions of the spectrum -- ordinary light, infrared, radio, ultraviolet -- are fundamentally the same. They are unified by thinking of them as "electromagnetic waves," waves of alternating strength in electric and magnetic fields that all move through space at the "speed of light" (called "c") of 300,000 kilometers per second (186,000 miles per second). The differing kinds of radiation simply have different wavelengths, (that is, the separations between crests in two successive waves).
Visual radiation is in the middle, with wavelengths that extend from 0.00004 centimeters for violet light to about 0.00007 centimeters for extreme red. These wavelengths are so short that astronomers use a small unit of distance, the "Angstrom" (A), which is 0.00000001 centimeters long. The violet limit therefore falls at 4000 A and the red limit near 7000 A or a bit longer. Infrared runs from the red limit to about 0.1 millimeter, and the radio to as long as you wish, even to kilometers. Ultraviolet runs from 4000 A down to about 100 A, X- rays take over to about 1 A, and these are followed by the gamma rays to no known lower limit. The named divisions are artificial and serve only to block out large spectral segments.
Molecules:
The electrons of two or more atoms can link together to form chemical bonds that make molecules from the chemical elements. The atoms can be the same or can be different.The molecules have characteristics that are completely different from their component atoms. There is no limit on the number of atoms that can be linked, and as a result there is an infinite number of kinds of molecules, the collection of which gives us all the riches of the natural world, including life.
A common example of a "chemical compound" is molecular oxygen (two oxygen atoms locked together) or water (two hydrogen atoms coupled with an oxygen atom).
Ions:
A normal atom, with equal numbers of protons and electrons, is electrically neutral. You get no electric shock by touching matter in its normal state. It is easy, however, to remove electrons from an atom and to electrically unbalance it. The result of electron removal is a positively charged "ion."
Isotopes:
The number of neutrons present in any kind of atomic nucleus is not fixed. All atoms have a set of variants called "isotopes" in which the proton number is the same but the neutron number is different. For example, the most common kind of hydrogen has only one proton. But you can attach a neutron to the proton and still have hydrogen. This heavy form, called "deuterium," is present in nature.
Monday, March 19, 2012
A Continuation on Radiation and Waves
As I stated before, temperature and radiation are very important in classifying stellar bodies. The temperature affects the type of element contained because each element burns differently, thus emitting different, measurable waves and kinds of radiation.
I also discovered that the temperature of stellar bodies affects what kinds of waves are emitted. As a general rule, it can be assumed that as the temperature of a stellar body (stars, nebulae, etc) rises; more radiation at all wavelengths is emitted moving towards shorter wavelengths.
So, as a result, these are the waves emitted at general temperatures:
I also discovered that the temperature of stellar bodies affects what kinds of waves are emitted. As a general rule, it can be assumed that as the temperature of a stellar body (stars, nebulae, etc) rises; more radiation at all wavelengths is emitted moving towards shorter wavelengths.
So, as a result, these are the waves emitted at general temperatures:
- Cold body: Radio waves
- Warm body: Infrared and radio waves
- Hot body: Visible (colors), infrared and radio waves
Monday, March 12, 2012
Atom Radiation
Today, I did a series of research into the radiation of different atoms, and their corresponding elements. I discovered that each different atom has a particular electron structure which can absorb a certain radiation energy level.
Another important point was that a star's temperature can be determined by the color because different radiation burns at certain temperatures, thus affecting color.
Reddish stars burn at 3000-4000 degrees Kelvin and Bluish stars burn at over 20,000 degrees Kelvin.
I also discovered that non-radioactive elements radiate based on the heat they contain. The kind of radiation in those elements depends on the temperature.
I plan on displaying this with a poster type presentation.
Another important point was that a star's temperature can be determined by the color because different radiation burns at certain temperatures, thus affecting color.
Reddish stars burn at 3000-4000 degrees Kelvin and Bluish stars burn at over 20,000 degrees Kelvin.
I also discovered that non-radioactive elements radiate based on the heat they contain. The kind of radiation in those elements depends on the temperature.
I plan on displaying this with a poster type presentation.
Monday, March 5, 2012
So it Begins...
Today in Physics class, I had an interesting time attempting to make this blog. In other words, it didn't go so well. But, that's all said and done and here we are.
For my Google inspired "20% Project," I will be looking at how stars and the elements in them are classified. This area of study is known as Stellar Classification. Based on the temperature of different stars and the elements they are made up of, different stars give off different colors and kinds of light waves, both visible and invisible.
Since I'm trying not to get too technical with this project (if at all possible), I would like to mainly investigate exactly how it is scientists deduce these things from stars. I also want to investigate the science surrounding Stellar Classification.
And now, on to research.
For my Google inspired "20% Project," I will be looking at how stars and the elements in them are classified. This area of study is known as Stellar Classification. Based on the temperature of different stars and the elements they are made up of, different stars give off different colors and kinds of light waves, both visible and invisible.
Since I'm trying not to get too technical with this project (if at all possible), I would like to mainly investigate exactly how it is scientists deduce these things from stars. I also want to investigate the science surrounding Stellar Classification.
And now, on to research.
Subscribe to:
Comments (Atom)