In the earlier part of the twentieth century, a discovery in quantum mechanics was made. It was discovered that the electrons that surround an atom have a minimum energy level that they will go to, or a minimum energy level. The electrons naturally seek their lowest energy levels. Since each minimum energy level is different for the electrons surrounding a specific atom or ion, different atoms absorb a specific energy level. However, electrons can be moved from one energy level to another through collisions or the absorption of photons, but an electron in a specific energy level is only capable of absorbing all or none of a photon, it cannot absorb a part of one. As a result, only photons with particular energies, those that correspond to the particular energy levels of the atom, can be absorbed from radiation. Also, since wavelength corresponds to photon energy levels, only specific wavelengths (or colors) can be absorbed.
Thus, since each atom or ion has a different electron structure, only specific wavelengths can be absorbed for that atom or ion.
When one looks at the (visible light/color) spectrum from a hot source (star, celestial body) after it has passed through low-density gas, narrow gaps at particular wavelengths where the light is diminished or even gone altogether are visible. Because of this appearance, these gaps or marks are called "absorption lines." Each atom or ion (of an element) has a unique set of absorption lines.
In stellar spectra, this technique can be used to discover the elements in stars. The deeper parts of stars contain large amounts of very hot, high-density gas and thus tend to radiate all the colors of the spectrum. However, the outer layers of stars are made up of a lower density gas and can act like the low-density gas in the earlier paragraph. Since stars are made up of the same chemical elements on earth (just in larger proportions), they can display an impressive mixture of absorption lines.The radiation from the center of the stars passes through the outer layer of gas allowing for scientists to read the resulting absorption lines. Those lines that are blacked out are the wavelengths that correspond to the elements in radiation.
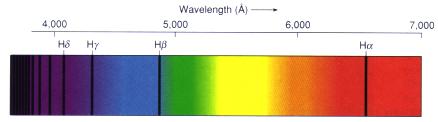
This is an example of the absorption lines of hydrogen against a continuous visual spectrum.
From "Astronomy! A Brief Edition," J. B. Kaler, Addison-Wesley, 1997.
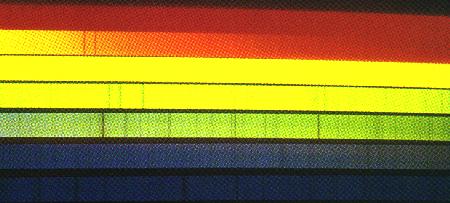
The solar spectrum is filled with absorption lines at particular colors or wavelengths, each dark line associated with a particular atom or ion. The pair in the orange, for example, are made by neutral sodium, the trio in the yellow by magnesium. Kitt Peak National Observatory.
No comments:
Post a Comment