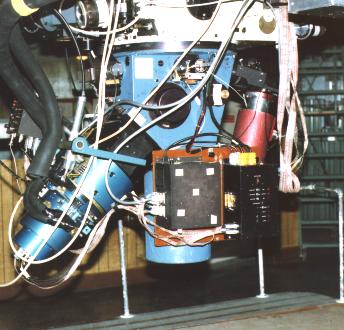
The spectrograph, fitted to the base of the telescope (seen at the top), breaks light into its component colors and records the spectrum. Light from a star goes straight to the collimator at the bottom of the instrument and is reflected back up to a diffraction grating in the middle. It is then reflected down and to the left to a digital detector for storage and display on a video terminal. University of Arizona Steward Observatory spectrograph photo by J. B. Kaler
However, more simple, "primitive" spectrometers involve little more than a prism. Most people know that shining a white light through a prism gives you a little rainbow: (This is because of dispersion).

Similarly, shining condensed light from a burning element, (or bit of the element with an electrical current running through it, as the device in my school's chemistry class does), through a prism will give you the parts of the "rainbow" for that element.
Example:

So, for my project I would like to attempt to make myself a little spectrometer with a prism (and only white light) to display how the technology works. These concepts also have a bit to do with color addition and subtraction, which I have been learning about in physics class recently.
The concept behind emission line and absorption lines goes off of the similar concepts of color addition and subtraction. In color subtraction, certain color wavelengths are absorbed so that others can be reflected. For example, a red shirt absorbs blue and green light and reflects only red. In absorption and emission lines, the electrons absorb certain wavelengths of color and reflect the others.
Color Subtraction:

Color Addition:

The primary light colors are: Red, Blue and Green.
The complementary light colors are: Magenta, Cyan and Yellow.
No comments:
Post a Comment