 In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result.
In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result. Followers
Monday, April 30, 2012
Experimentation
Today I had some fun searching the Physics classroom for a prism, which I eventually got my hands on. In my previous post, I showed how a simple spectrometer works. For my presentation, I plan on using this recently acquired prism in order to break up white light to show the basic concept. Since I don't have a camera handy right now, here's a picture of another person's prism set up.
 In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result.
In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result.
 In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result.
In order to break up the light, you shine a white light through a slit (in order to concentrate the light. I used a light box that my class has used for labs). Then, you shine the concentrated line of light through the prism, where a nice little rainbow will appear either on the ground or opposite structure at an angle from the prism. Lab use "prism spectrometers" work in a similar sense in that, instead of a white light, an element with an electric current running through it is shined at the prism, thus breaking the light and displaying only the colors the element "gives off." My personal rainbow was a lot weaker, but I'm going to experiment with brighter lights to see if I can get a better result. Saturday, April 28, 2012
The Spectograph
The tool used by scientists to see and record emission/ absorption spectra is called a Spectrograph or Spectrometer. Modern spectrographs, affixed to telescopes, look something like this:
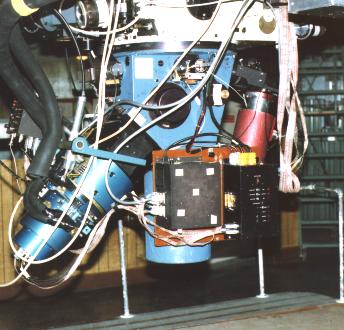





The spectrograph, fitted to the base of the telescope (seen at the top), breaks light into its component colors and records the spectrum. Light from a star goes straight to the collimator at the bottom of the instrument and is reflected back up to a diffraction grating in the middle. It is then reflected down and to the left to a digital detector for storage and display on a video terminal. University of Arizona Steward Observatory spectrograph photo by J. B. Kaler
However, more simple, "primitive" spectrometers involve little more than a prism. Most people know that shining a white light through a prism gives you a little rainbow: (This is because of dispersion).

Similarly, shining condensed light from a burning element, (or bit of the element with an electrical current running through it, as the device in my school's chemistry class does), through a prism will give you the parts of the "rainbow" for that element.
Example:

So, for my project I would like to attempt to make myself a little spectrometer with a prism (and only white light) to display how the technology works. These concepts also have a bit to do with color addition and subtraction, which I have been learning about in physics class recently.
The concept behind emission line and absorption lines goes off of the similar concepts of color addition and subtraction. In color subtraction, certain color wavelengths are absorbed so that others can be reflected. For example, a red shirt absorbs blue and green light and reflects only red. In absorption and emission lines, the electrons absorb certain wavelengths of color and reflect the others.
Color Subtraction:

Color Addition:

The primary light colors are: Red, Blue and Green.
The complementary light colors are: Magenta, Cyan and Yellow.
Emission Spectra
In my final presentation, I plan to explore and display a simple method for classifying elements by their wavelengths. This method is to look at a chemical element's emission spectra. Just like absorption lines, all chemical elements have a particular set of emission lines, which are essentially the opposite of absorption lines. Instead of measuring the energy/ photons absorbed by an electron, scientists measure and mark the energy radiated by electrons when they drop back down to low energies from "kicking" up to high energies. These lines appear as bright lines of color on a dark background instead of black, color-absent lines.
Emission lines are very easy to produce in a laboratory, which is one reason I am presenting them for my project. In the outside world, emissions are produced by street lamps, neon signs and florescent bulbs. In the wonderful world of space, nebulae and some stars can radiate emission lines as well.
Example:

Hydrogen emission lines are radiated by a hot, thin hydrogen gas, and appear at the same wavelengths as the hydrogen absorption lines.
From "Astronomy! A Brief Edition," J. B. Kaler, Addison-Wesley, 1997.
Interesting Find: Whilst looking around the internet for helpful information, I found this great simulation made by (I think) the University of Oregon. It allows you to look at the specific emission and absorption lines for every element on the periodic table and the corresponding wavelengths of each line.
Emission lines are very easy to produce in a laboratory, which is one reason I am presenting them for my project. In the outside world, emissions are produced by street lamps, neon signs and florescent bulbs. In the wonderful world of space, nebulae and some stars can radiate emission lines as well.
Example:

Hydrogen emission lines are radiated by a hot, thin hydrogen gas, and appear at the same wavelengths as the hydrogen absorption lines.
From "Astronomy! A Brief Edition," J. B. Kaler, Addison-Wesley, 1997.
Interesting Find: Whilst looking around the internet for helpful information, I found this great simulation made by (I think) the University of Oregon. It allows you to look at the specific emission and absorption lines for every element on the periodic table and the corresponding wavelengths of each line.
Monday, April 23, 2012
Composition of Stars
As I stated in the earlier post, stars are made up of the same chemical elements as earth, though in different amounts. Most stars are made up of about 90% hydrogen. They also contain helium. The rest of a star's composition is usually oxygen, followed by carbon, neon, and nitrogen. Of the metals stars contain, iron is the dominant element.
But, to put it in proportion, the Sun contains only one atom of oxygen for every 1200 atoms of hydrogen and only one iron atom for every 32 oxygen atoms.
However, there are many stars that do not follow this trend. Depending on their age or position in the Galaxy, it can deviate drastically.
These chemical elements are only discovered from the star's spectra.
But, to put it in proportion, the Sun contains only one atom of oxygen for every 1200 atoms of hydrogen and only one iron atom for every 32 oxygen atoms.
However, there are many stars that do not follow this trend. Depending on their age or position in the Galaxy, it can deviate drastically.
These chemical elements are only discovered from the star's spectra.
Monday, April 16, 2012
Absorption Lines
Now, to get to the roots of my earlier research. After looking at all that information about radiation and temperature, I had to read more about ions, molecules, isotopes and atoms to culminate in Absorption Lines!
In the earlier part of the twentieth century, a discovery in quantum mechanics was made. It was discovered that the electrons that surround an atom have a minimum energy level that they will go to, or a minimum energy level. The electrons naturally seek their lowest energy levels. Since each minimum energy level is different for the electrons surrounding a specific atom or ion, different atoms absorb a specific energy level. However, electrons can be moved from one energy level to another through collisions or the absorption of photons, but an electron in a specific energy level is only capable of absorbing all or none of a photon, it cannot absorb a part of one. As a result, only photons with particular energies, those that correspond to the particular energy levels of the atom, can be absorbed from radiation. Also, since wavelength corresponds to photon energy levels, only specific wavelengths (or colors) can be absorbed.
Thus, since each atom or ion has a different electron structure, only specific wavelengths can be absorbed for that atom or ion.
When one looks at the (visible light/color) spectrum from a hot source (star, celestial body) after it has passed through low-density gas, narrow gaps at particular wavelengths where the light is diminished or even gone altogether are visible. Because of this appearance, these gaps or marks are called "absorption lines." Each atom or ion (of an element) has a unique set of absorption lines.
In stellar spectra, this technique can be used to discover the elements in stars. The deeper parts of stars contain large amounts of very hot, high-density gas and thus tend to radiate all the colors of the spectrum. However, the outer layers of stars are made up of a lower density gas and can act like the low-density gas in the earlier paragraph. Since stars are made up of the same chemical elements on earth (just in larger proportions), they can display an impressive mixture of absorption lines.The radiation from the center of the stars passes through the outer layer of gas allowing for scientists to read the resulting absorption lines. Those lines that are blacked out are the wavelengths that correspond to the elements in radiation.
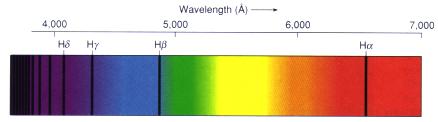
This is an example of the absorption lines of hydrogen against a continuous visual spectrum.
From "Astronomy! A Brief Edition," J. B. Kaler, Addison-Wesley, 1997.
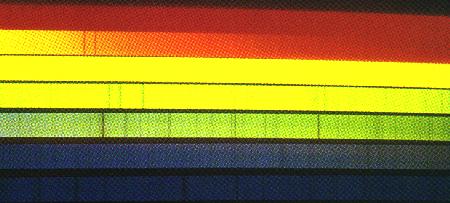
The solar spectrum is filled with absorption lines at particular colors or wavelengths, each dark line associated with a particular atom or ion. The pair in the orange, for example, are made by neutral sodium, the trio in the yellow by magnesium. Kitt Peak National Observatory.
In the earlier part of the twentieth century, a discovery in quantum mechanics was made. It was discovered that the electrons that surround an atom have a minimum energy level that they will go to, or a minimum energy level. The electrons naturally seek their lowest energy levels. Since each minimum energy level is different for the electrons surrounding a specific atom or ion, different atoms absorb a specific energy level. However, electrons can be moved from one energy level to another through collisions or the absorption of photons, but an electron in a specific energy level is only capable of absorbing all or none of a photon, it cannot absorb a part of one. As a result, only photons with particular energies, those that correspond to the particular energy levels of the atom, can be absorbed from radiation. Also, since wavelength corresponds to photon energy levels, only specific wavelengths (or colors) can be absorbed.
Thus, since each atom or ion has a different electron structure, only specific wavelengths can be absorbed for that atom or ion.
When one looks at the (visible light/color) spectrum from a hot source (star, celestial body) after it has passed through low-density gas, narrow gaps at particular wavelengths where the light is diminished or even gone altogether are visible. Because of this appearance, these gaps or marks are called "absorption lines." Each atom or ion (of an element) has a unique set of absorption lines.
In stellar spectra, this technique can be used to discover the elements in stars. The deeper parts of stars contain large amounts of very hot, high-density gas and thus tend to radiate all the colors of the spectrum. However, the outer layers of stars are made up of a lower density gas and can act like the low-density gas in the earlier paragraph. Since stars are made up of the same chemical elements on earth (just in larger proportions), they can display an impressive mixture of absorption lines.The radiation from the center of the stars passes through the outer layer of gas allowing for scientists to read the resulting absorption lines. Those lines that are blacked out are the wavelengths that correspond to the elements in radiation.

This is an example of the absorption lines of hydrogen against a continuous visual spectrum.
From "Astronomy! A Brief Edition," J. B. Kaler, Addison-Wesley, 1997.

The solar spectrum is filled with absorption lines at particular colors or wavelengths, each dark line associated with a particular atom or ion. The pair in the orange, for example, are made by neutral sodium, the trio in the yellow by magnesium. Kitt Peak National Observatory.
Subscribe to:
Comments (Atom)